Statement and Recommendations for Safety Assurance in Lung Ultrasound
Jul 16, 2023
Diagnostic ultrasound has made great strides since performance standards and recommended maximum exposure levels were set up in the 1980s and 90s. Lung ultrasound (LUS) has advanced to become an important clinical tool. The pleura appears in the image as a hyperechoic line, and defects in the line shown by image artifacts, such as B lines, are used to facilitate a variety of diagnoses in patients from neonates to high-BMI adults. In addition to the older methods for pulmonary effusion and thoracentesis, LUS has been found to be valuable in the diagnosis of pneumonia, pulmonary edema, pulmonary embolism, atelectasis, diffuse parenchymal disease, respiratory distress syndrome, COVID-19, and lung cancer.1–4 Lung ultrasund is particularly valuable in neonates at the point-of-care (POC) for diagnosis of respiratory distress syndrome,5 assessing surfactant treatment,6 pulmonary hemorrhage,7 and the number of B lines correlates with computed tomography findings,8 while avoiding any ionizing radiation exposure. An international review provides guidelines and 20 consensus statements on the use of LUS.9 Neonatal POC LUS has been found to be valuable in many patient settings, and is often routinely performed daily to follow patient progress.5,6 Neonatal exam guidelines noted the use of 10–15 MHz linear arrays at 10–12 positions on the neonatal chest, at birth and each week (ie, totaling 40–48 individual exams for scoring).10 Use of presets was recommended if available with adjustment of parameters to recommended settings.11 The concomitant evolution of ultrasound technology has provided simplified and handy devices that have revolutionized the practice of POC LUS, a major positive development in patient care.
The development of diagnostic ultrasound performance standards to clear diagnostic ultrasound machines for marketing in the United States began with the 1976 Medical Device Amendments to the 1938 Food, Drug, and Cosmetic Act. These standards aimed to ensure that new machines entering the market would be substantially equivalent in terms of safety and effectiveness to machines on the market prior to enactment of the 1976 Medical Device Amendments. The possibility of bioeffects of ultrasound was accepted, and a means to communicate ultrasound exposure levels to the practitioner was deemed essential. The National Equipment Manufacturers Association developed an Output Display Standard (ODS),12 with the aid of the American Institute of Ultrasound in Medicine, the US Food and Drug Administration, and the National Council on Radiation Protection and Measurements, among others, based on possible physical mechanisms for ultrasound bioeffects. The ODS is now maintained by the International Electrotechnical Commission.13 Two exposure indices were created to provide the practitioner with real-time exposure data. For the cavitational mechanism associated with pulse peak exposure, the Mechanical Index (MI) was defined as the derated peak rarefactional pressure amplitude pr (in MPa) in tissue divided by the square root of the ultrasonic frequency f (in MHz). pr is determined from ultrasound hydrophone measurements in water, then derated for tissue attenuation with a coefficient of 0.3 dB/cm-MHz (a conservative value for most tissues) with depth into the tissue. For the heat mechanism, associated with the spatial-peak temporal-average energy deposition, the Thermal Index (TI) provides an estimate of tissue heating, with three different TI conditions involving scanning paths with soft tissue (TIS), bone at the focus (TIB), and the cranium (TIC). The Output Display Standard, which included real-time display of MI and TI, provided a performance standard for the FDA’s regulatory review of new ultrasound imaging devices via a premarket notification pathway, also known as the 510(k) process.14 The required substantial equivalence to machines on the market prior to 1976 was accommodated by limiting the MI to 1.9 (determined from the maximum MI found in machines marketed prior to the enactment of the 1976 Medical Device Amendments).15 These indices are displayed on-screen for many machines, so that the practitioner can view and adjust the exposure as needed for optimal imaging and safety.
A unique biological effect of LUS is pulmonary capillary hemorrhage (PCH), which was discovered more than 30 years ago in mice,16 and has been confirmed in mice, rats, rabbits, pigs, and monkeys.17 This bioeffect has a deterministic response with identifiable exposure thresholds (unlike the x-ray stochastic response with no thresholds). Research with animal models of LUS has shown that the PCH bioeffect depends on pr, on specific pulsing parameters that change with the ultrasound mode, and on biological factors, including sedation, ventilation, age, lung position, and animal species.18 Lung ultrasound PCH cannot be explained only based on a thermal or a cavitational bioeffect mechanism.19 Above the threshold, PCH typically starts within seconds, increasing with exposure duration and is displayed as B lines on the image (analogous to B lines used for clinical diagnostic signs).20 The thresholds for PCH from research are advantageously related to the MI for clinical translation. Although the in situ MI thresholds measured in research may depend on various parameters, a conservative approach to fulfill the promise of doing no harm is to identify a worst-case minimum threshold for practitioner comparison to the on-screen MI. A consensus report and statement by the American Institute of Ultrasound in Medicine (AIUM)17,21 states that worst-case LUS exposure thresholds found for PCH are greater than MI=0.4 for B-mode imaging. This value has been specified in international guidelines and consensus reviews of LUS, and in best practice recommendations by the European Federation of Ultrasound in Medicine and Biology.9,22 Although this conclusion has not been contravened substantially in subsequent research, recent findings with diagnostic ultrasound machines indicate that the maximum number should be reduced slightly to MI ≤ 0.3.23,24 Fortunately, the use of lower MI settings can improve LUS imaging due to the reduction of saturation phenomenon for intense echoes from the pleura. One LUS guideline for standardized use recommends starting at MI=0.7 and reducing further for optimum imaging.4
The safety of neonatal LUS is of particular interest owing to the very thin chest walls and intensive clinical use. Age dependence was explored in early pulsed ultrasound research with mice, that indicated similar sensitivity for neonatal, juvenile, and adult mice.25 Pulmonary capillary hemorrhage depends on pr and the B mode imaging exposure threshold, expressed as the in situ MI, decreased modestly with increasing frequency from ≈0.80–0.84 at 1.5 MHz to ≈ 0.34–0.39 at 12 MHz, with a rat chest wall attenuation coefficient of 1.2 dB/cm-MHz.26 The tests with a linear array at 12 MHz had rats with 0.5-cm chest wall thickness (CWT), parameters similar to neonatal LUS. Pulmonary capillary hemorrhage results for neonatal swine (9 d old, 2.7 kg) with 0.78-cm CWT (swine CWT attenuation coefficient of 1.6 dB/cm-MHz) using a POC machine with curved linear array at 3.1 MHz were indicative of an in situ MI threshold of 0.53, and results with a linear array at 7.2 MHz were negative for in situ MI of 0.54.27 These results supported the similarity in animal models of LUS and emphasized the key role of the chest wall attenuation dependence on CWT and ultrasonic frequency in the occurrence of PCH.
Authoritative bodies provide safety recommendations to adjust the displayed MI ≤ 0.3.23,24 Paradoxically, this simple safety paradigm is impracticable to implement for the most widespread and beneficial POC LUS. Bioeffect safety education for practitioners can vary greatly by discipline, specialty, and program. The AIUM Medical Ultrasound Safety document26 is frequently provided with materials accompanying an ultrasound machine, but does not guarantee awareness by practitioners. There was no mention of the exposure level or MI in some LUS guidelines,11,29 and image figures included on-screen MI values of 1.2–1.5.29 Also, a standardization guide includes an MI=0.7 recommendation for avoiding image saturation and mentions potential lung damage.4 Surveys indicate a lack of knowledge for ultrasound safety assurance by many practitioners.30 In many LUS examinations, the POC practitioner may have no knowledge of the MI for the exam, why it is displayed (or sometimes not displayed), how to change it, nor why it might need to be changed for their patient.31,32 Additionally, many POC machines have no clear controls to change the MI or limitations on user adjustment. Ultrasound systems are also not required, as per the ODS, to display the MI if the machine is not capable of achieving a value greater than 1.0, thus complicating the practice of the as low as reasonably achievable (ALARA) safety principle for MI>0.3 during LUS. A new safety paradigm must be created for LUS.
Safety assurance strategies for MI>0.3 require the translation of the in situ MI provided in LUS PCH research to appropriate interpretation of the MI in the clinic. A key factor is the ultrasound attenuation by the chest wall (surface to pleura) thickness. A considerable safety margin can exist for the MI (relative to the actual in situ MI value) in many LUS exams owing to differences between the assumed attenuation coefficient for derating (0.3 dB/cm-MHz) and true tissue attenuation. An estimate of this margin can be made from simple ultrasonic wave exponential attenuation formulae by comparing the attenuation used for the MI to actual chest wall attenuation. The chest-wall-compensated mechanical index, MICWC, can be obtained by derating for chest wall attenuation AC instead of the MI derating value AMI throughout the CWT,
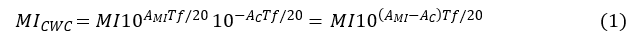
in which f is the working ultrasound frequency and T is the CWT (Equation 1). This formula provides an estimate that changes the safety assessment significantly by common changes in T and f, owing to the logarithmic exponential of base 10. Human intercostal tissue has an average attenuation coefficient, measured in adult tissue samples, of about 1.4 dB/cm-MHz,33 while the MI employs only 0.3 dB/cm-MHz. The attenuation adjustment (AMI–AC) in Equation 1 is then -1.1 dB/cm-MHz. The in situ exposure MI at the pleura generally will be less than that indicated by the MI, depending on CWT and ultrasonic frequency.
Neonatal patients have CWTs in the range of 0.2–0.8 cm.34 For a neonatal intercostal space of T=0.3 cm and f=10 MHz, assuming adult attenuation coefficient, MICWC (Equation 1) would be 0.68MI, affording little safety margin (ie, 1.3 for an MI = 1.9). As this most vulnerable patient population appears to be most at risk of PCH lung injury, problematic adjustment of settings would be needed to achieve MICWC ≤0.3. The POC-LUS practitioner should not be placed in the position of ensuring safety in neonates based on uncertain estimation of pleural exposure and repeated adjustments of machine output for every examination. Without appropriate presets there is increased risk of error and not maintaining an MI≤0.3. To compensate for operator variability and the increased risk of error associated with manual output adjustments, the practitioner’s responsibility for safe neonatal LUS should be achievable with the aid of a dedicated preset installed on the ultrasound machine.
Pediatric CWT values are typically 1–2 cm.35 An adult CWT varies, for example at the 5th intercostal space, from 2 cm (1.2–4.0 cm) for the 1st BMI quartile to 4.8 cm (2.5–10.3 cm) for the 4th BMI quartile.34 The average of about 3 cm can vary based on age, BMI, and intercostal space examined, with some patients having wall thicknesses above 10 cm. In an adult patient with T=3 cm and f=5 MHz, MICWC (Equation 1) would be 0.15MI, affording a significant safety margin. Even for the maximum recommended level of MI=1.9, MICWC would be reduced to an estimated in situ MI exposure value of only 0.29, which would satisfy the ≤ 0.3 safety value. It is important to keep in mind that such estimates are somewhat uncertain due to the unknown variation of the on-screen MI with image depth, focus, and other parameters, such as variations in attenuation coefficients for different patients. As such, there would be minimal concerns for many high BMI adult patients, for whom higher settings might be needed for optimal imaging.
Recommendations
A neonatal LUS safety preset incorporating a limit of MI≤0.3 should be pre-installed on all ultrasound machines. A clear means for the practitioner to select the LUS safety preset should be provided without the need for operator adjustment of output controls. Software updating to enable this neonatal safety preset should be programmed or installed in systems in current circulation.
In pediatric and adult patients, who have greater than neonatal chest wall thickness and variable safety margin, a LUS preset allowing higher displayed MI values should also be available when required for adequate imaging. Adherence to ALARA should be maintained with guidance from the consideration of chest wall attenuation, which will lower the MI exposure at the pleural surface.
The fixed limit for neonatal LUS is analogous to the fixed limit in the marketing clearance information for ophthalmic ultrasound (MI ≤ 0.23).14,15 The practice of ALARA for MI>0.3 should be aided by the consideration of MICWC values (Equation 1). Taken together, these recommended measures will help promote a practical safety paradigm for POC LUS. In the future, consideration should also be given to the development of a specific Mechanical Index for Lung (MIL, by analogy with the TI nomenclature) based on consideration of applicable nonthermal mechanisms, such as acoustical radiation pressure or force. This MIL should be displayed on-screen, during LUS, to provide a pleural exposure estimate with realistic CWT attenuation and updates with changing image parameters. Image-based automated programming also should be developed to automatically apply the optimum exposure for any LUS patient, for instance by detecting the depth of the pleural line, with possible quantitative imaging input, and adjusting the MIL accordingly, without the need for practitioner adjustments.
References
- Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012; 38:577–591.
- Soldati G, Demi M, Smargiassi A, Inchingolo R, Demi L. The role of ultrasound lung artifacts in the diagnosis of respiratory diseases. Expert Rev Respir Med 2019; 13:163–172.
- Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung ultrasound for critically ill patients. Am J Respir Crit Care Med 2019; 199:701–714.
- Soldati G, Smargiassi A, Inchingolo R, et al. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19: a simple, quantitative, reproducible method. J Ultrasound Med 2020; 39:1413–1419.
- Xin H, Wang L, Hao W, Hu H, Li H, Liu B. Lung ultrasound in the evaluation of neonatal respiratory distress syndrome. J Ultrasound Med 2022; 42:713–721.
- Razak A, Faden M. Neonatal lung ultrasonography to evaluate need for surfactant or mechanical ventilation: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2020 Mar;105(2):164-171.
- Corsini I, Parri N, Ficial B, Dani C. Lung ultrasound in the neonatal intensive care unit: Review of the literature and future perspectives. Pediatr Pulmonol 2020; 55:1550–1562.
- Martelius L, Heldt H, Lauerma K. B-lines on pediatric lung sonography: comparison with computed tomography. J Ultrasound Med 2016; 35:153–157.
- Demi L, Wolfram F, Klersy C, et al. New international guidelines and consensus on the use of lung ultrasound. J Ultrasound Med 2023; 42:309–344.
- Loi B, Vigo G, Baraldi E, et al. Lung ultrasound to monitor extremely preterm infants and predict bronchopulmonary dysplasia. A multicenter longitudinal cohort study. Am J Respir Crit Care Med 2021; 203:1398–1409.
- Liu J, Guo G, Kurepa D, et al; Society of Pediatrics, Asia-Pacific Health Association; the Division of Critical Ultrasound, Pediatric Society of Asia-Pacific Health Association; the Critical Ultrasound Group of Neonatal Specialty Committee, the Cross-Straits Medicine Exchange Association as well as the World Interactive Network Focused On Critical Ultrasound China Branch. Specification and guideline for technical aspects and scanning parameter settings of neonatal lung ultrasound examination. J Matern Fetal Neonatal Med 2022; 35:1003–1016.
- Real-time Display of Thermal and Mechanical Acoustic Output Indices on Diagnostic Ultrasound Equipment. NEMA UD 3-2004.
- IEC, Particular Requirements for the Basic Safety and Essential Performance of Ultrasonic Medical Diagnostic and Monitoring Equipment. IEC 60601-2-37: 2007+AMD1:2015 CSV, International Electrotechnical Commission, Geneva, Switzerland.
- Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. U.S. Department of Health and Human Services. Food and Drug Administration, Center for Devices and Radiological Health; 2019.
- Nyborg WL. Biological effects of ultrasound: development of safety guidelines. Part II: general review. Ultrasound Med Biol 2001; 27:301–333.
- Child SZ, Hartman CL, Schery LA, Carstensen EL. Lung damage from exposure to pulsed ultrasound. Ultrasound Med Biol 1990; 16:817–825.
- Church CC, Carstensen EL, Nyborg WL, Carson PL, Frizzell LA, Bailey MR. The risk of exposure to diagnostic ultrasound in postnatal subjects: Nonthermal mechanisms. J Ultrasound Med 2008; 27:565–592.
- Miller DL, Abo A, Abramowicz JS, et al. Diagnostic ultrasound safety review for point-of-care ultrasound practitioners. J Ultrasound Med 2020; 39:1069–1084.
- Miller DL. Mechanisms for induction of pulmonary capillary hemorrhage by diagnostic ultrasound: review and consideration of acoustical radiation surface pressure. Ultrasound Med Biol 2016; 42:2743–2757.
- Miller DL, Dong Z, Dou C, Raghavendran K. Influence of scan duration on pulmonary capillary hemorrhage induced by diagnostic ultrasound. Ultrasound Med Biol 2016; 42:1942–1950.
- AIUM Official Statements: Statement on Biological Effects of Ultrasound in Vivo. American Institute of Ultrasound in Medicine website. https://www.aium.org/resources/official-statements/view/statement-on-biological-effects-in-tissues-with-ultrasound-contrast-agents-2. Accessed August 2021.
- Wolfram F, Miller D, Demi L, et al. Best practice recommendations for the safe use of lung ultrasound. [published online ahead of print March 3, 2023]. Ultraschall Med. doi: 10.1055/a-1978-5575.
- BMUS. Guidance document on ultrasound safety issues when scanning a neonate. Physics and Safety Committee of the British Medical Ultrasound Society website. BMUS.org. Accessed October 2021.
- Sande R, Jenderka KV, Moran CM, et al. Safety aspects of perinatal ultrasound. Ultraschall Med 2021; 42:580–598.
- Dalecki D, Child SZ, Raeman CH, Cox C, Penney DP, Carstensen EL. Age dependence of ultrasonically induced lung hemorrhage in mice. Ultrasound Med Biol 1997; 23:767–776.
- Miller DL, Dou C, Raghavendran K. Dependence of thresholds for pulmonary capillary hemorrhage on diagnostic ultrasound frequency. Ultrasound Med Biol 2015; 41:1640–1650.
- Miller DL, Dou C, Dong Z. Lung ultrasound induction of pulmonary capillary hemorrhage in neonatal swine. Ultrasound Med Biol 2022; 48:2276.
- AIUM. Medical Ultrasound Safety 4th Edition. Laurel, MD: American Institute of Ultrasound in Medicine. AIUM.org; 2020.
- Kurepa D, Zaghloul N, Watkins L, Liu J. Neonatal lung ultrasound exam guidelines. J Perinatol 2018; 38:11–22.
- Lalzad A, Wong F, Singh N, et al. Knowledge of safety, training, and practice of neonatal cranial ultrasound: a survey of operators. J Ultrasound Med 2018; 37:1411–1421.
- Abramowicz JS. Biosafety of sonography: still a mystery to most obstetrics (and other) providers. J Ultrasound Med 2020; 39:1683-1685.
- Abramowicz JS. Bioeffects and safety of lung ultrasound in neonates. J Ultrasound Med 2022; 41:1031.
- Patterson B, Miller DL. Experimental measurements of ultrasound attenuation in human chest wall and assessment of the mechanical index for lung ultrasound. Ultrasound Med Biol 2020; 46:1442–1454.
- Goelz R, Krumrey S, Dietz K, Esser M, Poets CF. Safely inserting neonatal chest drains. Neonatology 2022; 119:33–40.
- Terboven T, Leonhard G, Wessel L, et al. Chest wall thickness and depth to vital structures in paediatric patients - implications for prehospital needle decompression of tension pneumothorax. Scand J Trauma Resusc Emerg Med 2019; 27:45.
- Inaba K, Ives C, McClure K, et al. Radiologic evaluation of alternative sites for needle decompression of tension pneumothorax. Arch Surg 2012; 147:813–818.
